
There are approximately 275 different isotopes of 81 stable elements. Isotopic forms of Carbon-Carbon– 12, Carbon – 13, Carbon- 14.Hydrogen – 1, Hydrogen – 2, Hydrogen – 3 are the isotopic forms of Hydrogen.Isotopic forms of Fluorine – Fluorine 17, Fluorine 18, Fluorine 19.Chlorine- 35, Chlorine – 37 are the isotopic forms of chlorine.Isotopic forms of Uranium- U-235, U-238.Isotopic forms of Oxygen – Oxygen -16, Oxygen -17, Oxygen -18.Carbon-12 is the stable isotope of the carbon element whereas carbon-14 is the radioactive isotope. Therefore, the atomic number 6 of carbon in all the forms is constant. As discussed, atomic number is the unique property by which we can determine the element. The numbers 12, 13, and 14 represents the atomic masses of different isotopic forms of carbon. The number of neutrons in protium is zero, the number of neutrons in deuterium is one and the number of neutrons in tritium is two.Ĭarbon has three isotopic forms- Carbon-12, Carbon-13, as well as Carbon-14.
All the three isotopic forms of hydrogen have the same number of protons but vary in the number of neutrons. Hydrogen has three most stable isotopic forms- protium, deuterium, as well as tritium. Thus, the definition “ Elements with the same atomic number but a different mass number are termed as Isotopes.” For Example The number of neutrons varies but the number of protons always remains same in an isotope of a single element. However, the number of neutrons can change. In a certain element, the number of protons will always remain constant. The number of protons and neutrons combined together is called atomic mass or mass number of an element, whereas the total number of protons gives the atomic number of an element.
#STRUCTURE OF ISOTOPES OF CHLORINE DOWNLOAD#
You can download Structure of Atom Cheat Sheet by clicking on the download button belowĮlements with the same atomic number but a different mass number are defined as “Isotopes”. Towards Quantum Mechanical Model of Atom.Development Leading to Bohr’s Model of Atom.How are Electrons Distributed in Different Orbits (Shells)?.Browse more Topics Under Structure Of Atom
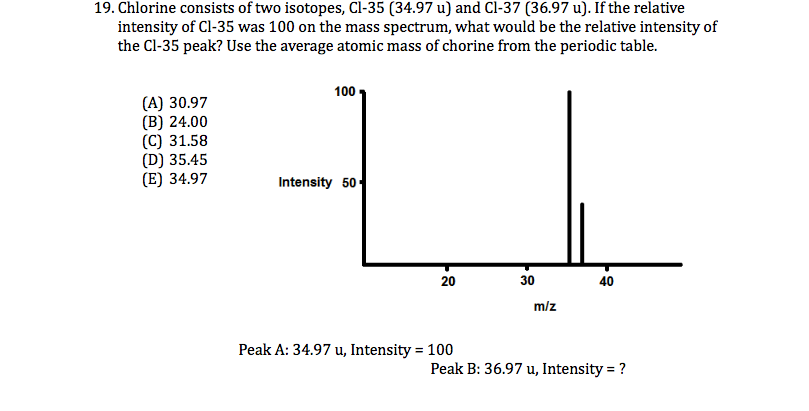
Read through this article to find out more about isotopes.
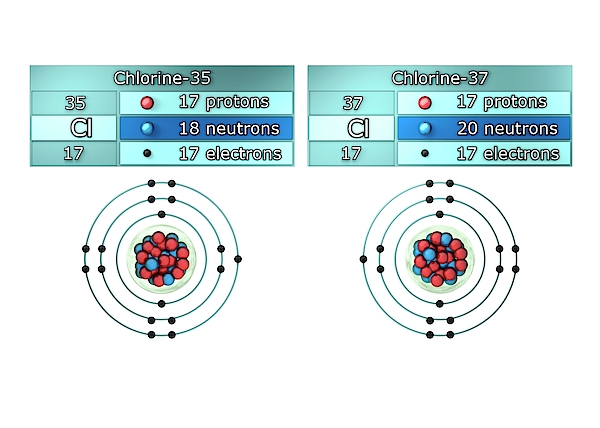
These different varieties of the same element are termed isotope. The number of neutrons in both the varieties will be different. Elements or atoms are present both in radioactive and non-radioactive variety. Thus it has a tendency to release subatomic particles to obtain stability thereby releasing radiation or energy in the process. Every radioactive atom will have an unstable nucleus. Radioactivity is one of the properties of atoms.


 0 kommentar(er)
0 kommentar(er)
